The Problem
In 2012 a counterfeit of the cancer drug Avastin was found in the United States, entering the country through a complex worldwide “grey market” of suppliers. The counterfeit drugs traced a path through Turkey, Switzerland, the United Kingdom, and finally the United States, ending up in the hands of cancer patients fighting for their lives. Unbeknownst to the medical professionals prescribing what they thought were lifesaving prescriptions, the drugs were devoid of the active ingredient needed to reduce the growth of patients’ tumors.[1] The World Health Organization estimates that there are over 1 million deaths annually from counterfeit and substandard drugs, causing $21 billion global financial impacts.[2] For the past 10 months, recalls of blood pressure medications tainted with a known carcinogen have continued to expand, impacting tens of millions of patients in the United States.
Gaps in the Pharmaceutical Supply Chain
The worldwide and complex nature of the global pharmaceutical supply chain provides ample opportunities for counterfeit and substandard drugs to enter the legitimate supply chain. Failure to use manufacturing best practices, rigorous quality control testing, and proper temperature controls during shipments, are common ways that substandard drugs to enter the supply chain. Counterfeit drugs rarely enter the legitimate supply chain in the United States. However, the emergence of online pharmacies present a unique challenge in combating counterfeit drugs, as an already complex network of suppliers becomes even more opaque with the involvement of fraudulent or unlicensed online pharmacies that attract consumers looking for privacy or cheaper prices.[3]
With our current pharmaceutical tracking systems, it’s nearly impossible to determine where drugs originated or to be 100% sure they are safe. Pharmaceutical supply chains often use manual procedures and paper-based systems to track pharmaceuticals. Even if the tracking data is stored electronically it’s generally siloed with the manufacturer, shipper, or warehouse, rather than shared throughout the supply chain. With the bulk of tracking information being included as a label on the drum, pallet, or container, this makes it relatively easy to add counterfeit drugs into the supply chain. In the event a recall is issued, special firms are often brought in to aggregate the disparate tracking mechanisms; adding precious time to complete the recall. [4]
Where Blockchain Can Help
Blockchain may provide an ideal solution to reduce the instances of counterfeit and substandard drugs. Blockchain is a distributed ledger technology that connects transactions in a way that they cannot be edited or changed after being entered into the blockchain. A cryptographic code, called a hash, is permanently attached to all transactions- ensuring that once the transaction has been entered, it’s there for good. Both financial transactions and other transaction types such as shipping information and temperature data can be entered into the blockchain. Additionally, each participant in the blockchain network participates in validating each transaction ensuring that no individual participant is editing or adding to the blockchain without proper validation of the entry.
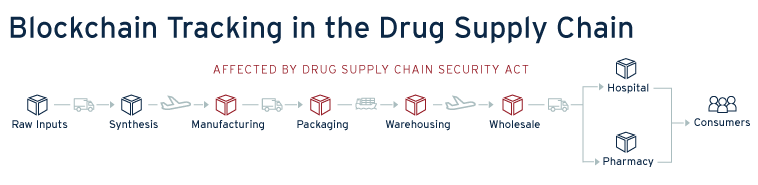
The immutability and network validation provide a compelling case for this technology to be used in combating counterfeit or substandard drugs. If a pharmaceutical supply chain was built around, and tracked with blockchain, everytime a drug was touched from creation, to shipment, registration in storage and the sale, each transaction would be entered and tracked in the blockchain. This would provide an incredibly transparent tracking mechanism for drug validity and security. Additionally, in the case of a recall the pharmaceutical company would have a direct and centralized means of tracking its supply chain in order to issue and expedite recalls; dramatically curtailing timelines. With the transparency provided by blockchain, participants within the supply chain would be incentivized to do better testing, provide better temperature control, and ensure the validity of their drugs, as the blockchain tracking system would give immediate tracing capability to downstream participants in the chain. Additionally, this visibility could allow pharmacists or prescription recipients to look at the supply chain data providing confidence in their medicine.
Blockchain To Meet Tracking Requirements
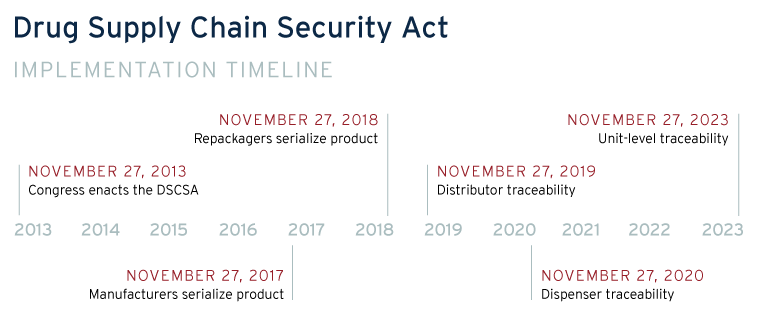
In an important step towards better visibility in the pharmaceutical supply chain, the Drug Supply Chain Security Act (DSCSA), passed in 2013, charged the Federal Drug Administration “build an electronic, interoperable system to identify and trace certain prescription drugs as they are distributed in the United States”. [5] The first move toward implementation has been to add individual identifiers to certain drugs, a potential integration point with a blockchain tracking system. Additionally, the DSCSA requires that tracking occur and chain of custody is kept between manufacturers, transporters, storage facilities, and dispensers. Blockchain is an ideal means to meet the tracking requirements of the DSCSA, providing an immutable recordkeeping system and providing immediate visibility throughout the supply chain. If issues were found in the supply chain the blockchain system’s immediate visibility would help achieve the 24 hour notification requirement of the DSCSA.
Saving Lives and Saving Money
Implementing a blockchain tracking system has the potential to reduce the human costs and the immense drug recall costs associated with counterfeit and substandard drugs. The settlements associated with the recall of Vioxx, a drug produced by Merck, were $4.85 billion and the recall of Bextra, a drug produced by Pfizer, is estimated to have cost the company about $3 billion.[6] If implementing a blockchain solution in these instances could have reduced the costs of these recalls even by a few percent, it would have saved hundreds of millions of dollars for these companies, sparing innocent lives in the process.
Getting Started
Under DSCSA, the Federal Drug Administration (FDA) has until 2024 to fully implement all requirements of the Act. Recently, the FDA began piloting blockchain as a test case digital tracking system. As evidenced by the IBM Food Trust, blockchain has been successful in tracking complex food supply chains especially with a major market player like Walmart requiring their suppliers to adopt the technology.[7]Ideally, under the DSCSA requirements, we’ll see large pharmaceutical companies begin to adopt blockchain at a larger scale, using their market influence to foster adoption. Startup companies such as Mediledger, Farmatrust, and AMBROSUS have begun to build and implement blockchain tracking platforms for pharmaceuticals.
As blockchain is beginning adoption in the United States, the true measure of success will be how the models develop and how lessons learned will be applied globally to developing nations where drug counterfeiting issues are much more severe. Only once we begin to provide true visibility into the global drug supply chain, will these efforts realize their full value. We believe that implementing an unalterable tracking system through blockchain is a major step in the right direction towards reducing the massive human cost of counterfeit and substandard drugs.
[1]Mackey, T. K., Cuomo, R., Guerra, C., & Liang, B. A. (2015). After counterfeit avastin®--what have we learned and what can be done?Nature Reviews.Clinical Oncology, 12(5), 302-308. doi:http://dx.doi.org.proxyau.wrlc.org/10.1038/nrclinonc.2015.35
[2]World Health Organization, “Substandard and Falsified Medical Products”, https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products, (January 31, 2018)
[3]Institute of Medicine of the National Academies,Countering the Problem of Falsified and Substandard Drugs, (February, 2013)
[4]E. W. Schuster and R. Koh. Track and Trace in the Pharmaceutical Supply Chain. Auto-ID Labs, Massachusetts Institute of Technology Cambridge, MA.
[5]https://www.fda.gov/drugs/drugsafety/drugintegrityandsupplychainsecurity/drugsupplychainsecurityact/